In metal foundries, aluminum and its alloy are among the top casting materials due to their exceeding advantages over other metals. Aluminum alloy has ductility, high castability, high strength, and especially, high corrosion resistance in different environments and chemical agents.
Aluminum’s corrosion resistance is created by an inert oxide film forming on a metal surface that provides a protective layer, preventing the aluminum surface from exposure to its surroundings.
An oxide film on the surface is formed due to the aluminum surface’s chemical reaction with oxygen and water. In fact, this is the first stage of metal corrosion. Although the oxide film is only 5-10 nm thin, it prevents metal rust as soon as it is exposed to an oxidizing environment like water.
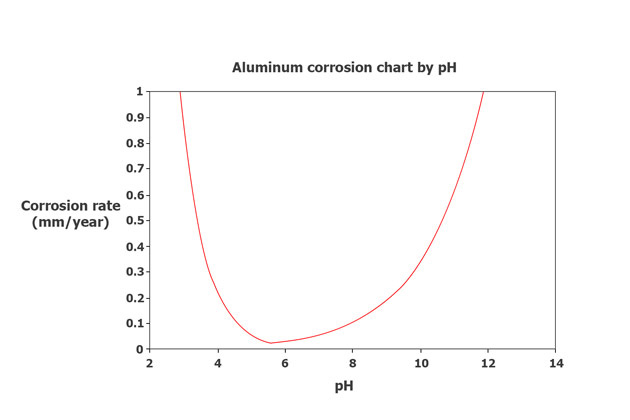
In most environments, the corrosion rate of aluminum alloys decreases over time partly because the oxide film’s stability determines its resistance to corrosion, which depends on the pH value of the environment.
Typically, oxide films are stable in the pH range of about 4 to 8. If the pH is below 4, it will dissolve the acids, and if the pH is above 8, it will dissolve alkali to create crystalline solids, which is rust.
In the casting industry, in order to improve the casting properties of aluminum and reduce aluminum casting defects, metal foundries often add a higher Si content to the aluminum alloy, which makes localized corrosion more likely to happen and discourages the anodizing to lower the corrosion resistance of the cast aluminum.
In order to optimize the corrosion resistance of cast aluminum alloys, it is necessary to understand the relationship between the aluminum alloy production process and the microstructure of aluminum. Therefore, we conducted a comprehensive assessment of the research on the corrosion of metals in general and aluminum alloys in particular according to several aluminum casting methods, such as sand casting, low pressure die casting, etc.
This is the most detailed, comprehensive, and straightforward article you can find about aluminum alloys’ corrosion, which is highly reactive and have various outstanding properties.
Let’s take a closer look with us – VIC casting foundry!
- Corrosive nature of aluminum alloys
- Corrosion limit of aluminum alloys
- 1. The 1xx.x series alloy: Commercially pure Aluminium
- 2. The 2xx.x series alloy: Aluminum – copper
- 3. The 3xx.x series alloy: Aluminum – Manganese
- 4. The 5xx.x series alloy: Aluminum – Magnesium
- 5. The 6xx.x series alloy: Aluminum – Magnesium – Silicon
- 6. The 7xx.x series alloy: Aluminum – Zinc – Magnesium
- 7. The 8xx.x series alloy: Aluminum – Lithium
- 8. The 9xx.x series alloy: Aluminum – Nikel
- Effect of alloying elements on aluminum alloy corrosion
- 1. The effect of Mg
- 2. The effect of Si
- 3. The effect of Cu
- 4. The effect of Zn
- 5. The effect of Fe
- 6. The effect of Mg
- 7. The effect of Li
- Types of aluminum alloy corrosion
- Uniform corrosion
- Galvanic corrosion
- Crevice Corrosion
- Pitting corrosion
- Intergranular Corrosion
- Exfoliation Corrosion
- Stress Corrosion Cracking
- Corrosion Fatigue
- Filiform Corrosion
- Aluminum corrosion resistance
- Conclusion
Corrosive nature of aluminum alloys

Corrosion of aluminum alloys in a substantial environment is due to the cathodic and anodic reactions co-occurring at the same rate on the metal surface.
The cathodic reaction is the oxidation process of the metal, and the anodic reaction is the reduction of the substance in the environment. Oxidation and reduction happen simultaneously, and electrons are transferred between the two reactants. Therefore, the metal carries electricity.
Oxidation reaction: Al → Al3+ + 3e
Hydrogen redox reaction: H+ + e → ½ H2
Or oxygen redox reaction: O2 + 2H2O + 4e–→ 4OH–
In essence, these reactions occur in the microstructure of the alloy. The microstructure of an aluminum alloy is determined by the alloy elements and by the thermomechanical treatment.
With pure aluminum alloy with no metallic elements added, there are no metal positions inside the microstructure. As a result, the cathodic reaction is unlikely to happen, thereby minimizing the corrosion possibility.
Regarding heterogeneous aluminum alloys mixed with alloying components, intermetallic particles are formed to produce 1-300 nm in diameter precipitates. The precipitates consist of different electrochemical characteristics that are the areas being attacked by corrosion.
Corrosion limit of aluminum alloys
Aluminum’s resistance to corrosion increases as its purity increases. However, due to aluminum’s high ductility, the purer the aluminum alloy is, the fewer applications it provides.
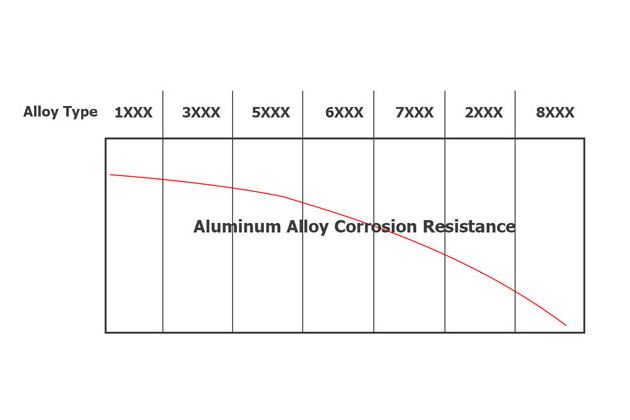
Typically, metals are added to increase aluminum’s hardness and castability, leading to a decrease in its corrosion resistance.
Below are analyzes of aluminum alloys’ corrosion resistance.
1. The 1xx.x series alloy: Commercially pure Aluminium
The 1xx.x series alloy is the purest alloy, containing about 99.93% pure aluminum, and it has a very low measured corrosion rate, about 0.8 µA in 1 cm2 to 2.3 cm2.
Due to the excellent corrosion resistance, the 1xx.x series is not widely applied daily due to its low hardness.
Some of its applications include 100 series alloys used in the foil packaging industry and as a material for cookware. It is also used to produce secondary alloy production or as a coating for other series.
2. The 2xx.x series alloy: Aluminum – copper
2xx.x series aluminum alloy contains a high content of Cu, about 4-10%, so it has high mechanical properties and is used in structures, especially in the aerospace construction industry.
However, the addition of Cu to the alloy will affect its durability. Although the hardness is significantly improved (about 500Mpa), it is susceptible to corrosion in industrial environments with humidity.
The 200 series tends to form casting defects so they are often limited to the production of casting simple patterns.
An electrochemical test with a 0.5M H2SO4 solution measured the corrosion rate of about 0.45µA/cm2, compared with measurement in 3% NaCl solution. This experiment was conducted with three Al-Cu alloys with Cu ratio of 5%, 10%, and 15%, respectively, and it is concluded that the corrosion rate of 3 alloys was the same.
Another experiment was conducted using the directional solidification method, researching the effect of the cooling rate and the Cu content of Al-4.5%Cu alloy.
Three samples were taken at three different positions from the metal-cooler surface. Observing at the microscopic level, shows a better corrosion rate when the cooling rate is higher.
3. The 3xx.x series alloy: Aluminum – Manganese
The 3xx.x series aluminum alloy is usually available in thin sheets. It is an aluminum alloy with the addition of silicon and about 1% manganese component to improve corrosion resistance in the solid solution. The strength of this alloy is average, about 110MPa.
If cold work and annealing are performed, the 3xxx series will achieve excellent mechanical properties. They also have high casting properties, so up to 90% of the world cast aluminum belongs to the 300 series. Therefore, corrosion research with cast aluminum alloys is usually conducted on series 300.
Many experiments on 3xx.x series alloy corrosion have been conducted, mainly with gravity casting technology and pressure die casting.
Aluminum alloy casting by gravity casting technology
A study showed that the corrosion current density and the impedance parameter of Al-8%Cu-3%Si induce a higher corrosion resistance than the Al-6%Cu-1%Si.
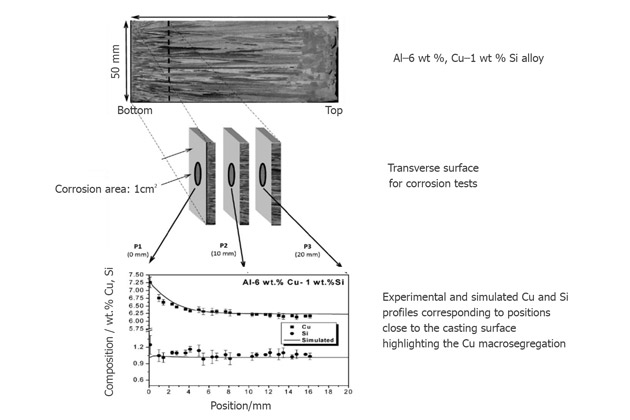
Corrosion tests are performed at the casting cross-section position, at 0, 10, and 20mm from the cold casting surface.
This experiment shows that Si and secondary dendrite arm spacing (SDAS) content depend on each other and affect the corrosion resistance of Al-6%Cu-1%Si.
In the α aluminum matrix, cast aluminum alloys easily form intermetallic compounds. The presence of manganese in the 300 series compensates for Fe’s cathodic effect in intermetallic compounds, making them less corrosive.
Researchers also conducted experiments on the effects of Sr on the corrosion of aluminum alloys.
Micro-observations further showed the change of silicon eutectic from coarse and thin morphology to the connective fiber morphology.
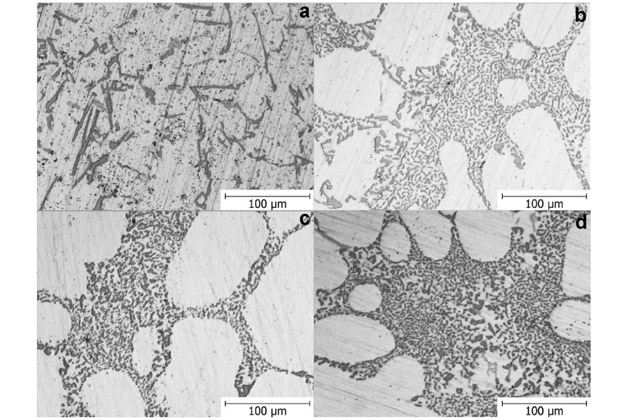
a. no Sr added
b: Sr 120 ppm
c: Sr 170 ppm
d: Sr 250 ppm
Conclusion: The bonded fiber morphology of eutectic silicon enhances the corrosion resistance of aluminum alloys. Detailed levels of corrosion resistance are shown in the following table:
| Sr addition | Before | After |
| 120 ppm | 13.8 µA/cm2 | 0.42 µA/cm2 |
| 150 ppm | 10.2 µA/cm2 | 1.47 µA/cm2 |
Aluminum alloy casting with HPDC technology (high pressure die casting)
According to the recorded documents, this experiment sprayed metal at three different temperatures: 579°C, 643°C, and 709°C with two injection pressures of 35 MPa of 70 MPa. Microstructure observation shows that at low temperature, the dendrite of aluminum is fragmented, and at high temperature, dendrite is more refined.
This experiment concludes that the higher the porosity, the lower the aluminum alloy’s corrosion resistance. The porosity increases gradually according to the following experimental results:
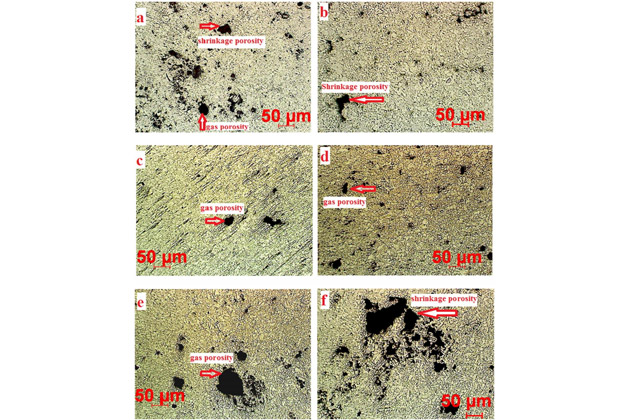
a: 579°C / 35 MPa (3.15% porosity)
b: 579°C / 70 MPa
c: 643°C / 35 MPa
d: 643°C / 70 MPa
e: 709°C / 35 MPa
f: 709°C / 70 MPa (4.91% porosity)
With HPDC technology, air bubbles are created due to high injection rate, resulting in high porosity casting and causes gas porosity defects.
4. The 5xx.x series alloy: Aluminum – Magnesium
5xx.x series contains less than 6% Mg. Magnesium has a solubility in aluminum that enhances the alloy’s corrosion resistance and hardness. The hardness of series 500 is higher than 380MPa.
500 series can resist corrosion in seawater environment, so it is applied in the marine industry.
Research on corrosion resistance of Al-3%Mg-1%Si alloy by gravity casting and continuous casting method found severe corrosion at the precipitate free zone.
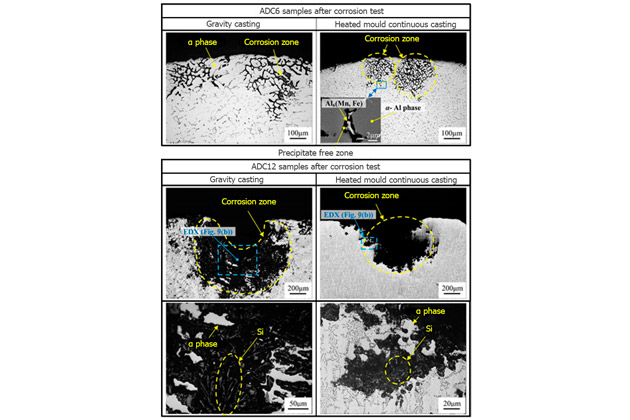
The above figure shows the experiment results; with gravity casting technology, Al-3Mg-1Si alloy is corroded at a higher rate than continuous casting. In fact, continuous casting technology has a faster cooling rate, leading to a higher solid solution ratio.
The 500 Series also has a corrosion problem – a heavy density disorder that can cause harmful β-phase Mg2Al3 precipitation on alloys with > 3% Mg, and high-temperature exposure for long periods.
5. The 6xx.x series alloy: Aluminum – Magnesium – Silicon
Silicon-based 6xx.x series aluminum alloy increases fluidity and reduces melting point.
This alloy has a hardness strength > 300MPa, mainly in extruded form and in sheet form.
Silicon and Mg are added to the alloy, exceeding 1.4% will increase the strength when aging.
600 series obtain good corrosion resistance, so it is widely used in the marine environment and train engine manufacturing.
6. The 7xx.x series alloy: Aluminum – Zinc – Magnesium
7xx.x series alloy has durability up to 580MPa; such high strength is achieved due to the η-phase (MgZn2) precipitation. Therefore, it is widely used in the aerospace industry.
The 700 series’ disadvantage is the reduced corrosion resistance; they are susceptible to environmental erosion and stress corrosion cracking.
To rebalance the corrosion resistance, complex heat treatments were performed, such as secondary heat treatments.
7. The 8xx.x series alloy: Aluminum – Lithium
8xx.x series aluminum alloy is mixed with Li element, with the solubility in aluminum up to 16%.
The 800 series is very light weight and obtain high rigidity; thus, it has a high potential for aerospace industry usage.
In the past, Li-containing aluminum alloys had the highest corrosion rate among all aluminum alloys; but today, Al-Li alloys with added Cu have overcome this limitation.
8. The 9xx.x series alloy: Aluminum – Nikel
9xx.x series alloy with Nickel added to increase hardness but reduce ductility and resistance to corrosion.
A study on Al-5%Ni alloys: took 1 sample of the alloy at position P1, which is 10mm apart from the edge of the mold with a cooling rate of about 8°C/s, and 1 sample at 60mm apart from the mold edge at a cooling 0.6°C/s.
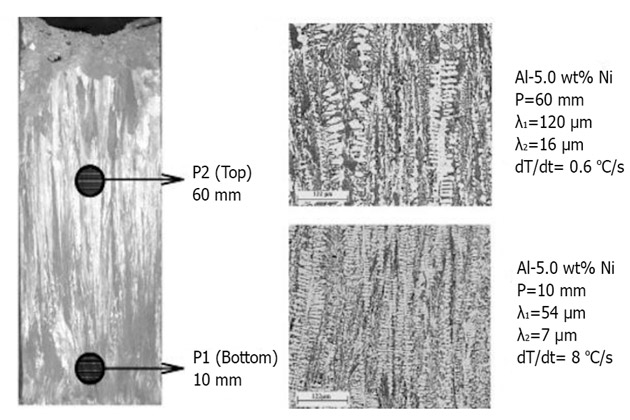
The result was the corrosion rate of position P2 is 1.5 µA/cm2, and in position P1 is 3.5 µA/cm2.
Find out more information about types of aluminum: https://vietnamcastiron.com/types-aluminum/
Effect of alloying elements on aluminum alloy corrosion
1. The effect of Mg
Mg is added to aluminum alloy to improve mechanical properties. Mg reduces the reaction rate of cathodic reaction when present in solid solution (due to low exchange current density of Mg) and increases corrosion resistance.
2. The effect of Si
Si is added with Mg creates a Mg2Si precipitate that enhances the aluminum alloy’s hardness but causes local corrosion. Adding excessive Si will cause stress corrosion cracking due to Si appearing at the boundary and speed up the cathodic reaction.
3. The effect of Cu
Similar to Mg, the presence of Cu causes an aluminum alloy to form a localized cathodic reaction that causes corrosion. However, 600 or 700 series alloys’ principal aim at adding Cu to the composition for strengthening hardness purpose, not for the anti-corrosion goal.
4. The effect of Zn
Adding Zn to the aluminum alloy may form τ phase Al-Mg-Zn instead of β phase Al3Mg2, which causes stress corrosion cracking. Alloys used in the aerospace industry still use Zn to form a hardness-enhanced precipitate.
5. The effect of Fe
In the production process, aluminum alloy often mixes Fe into the composition. The Fe removing process is very costly. Fe is difficult to dissolve in the alloy and maintains a cathodic reaction, resulting in reduced corrosion resistance. Fe combined with Mn or Cu in the alloy is also a factor preventing corrosion resistance.
6. The effect of Mg
Including Manganese in the aluminum alloy will reduce Fe concentration and increase corrosion resistance. However, if the amount of Mn exceeds the solubility limit (1.25% by weight), it will lead to the formation of Al6Mn, which increases the cathodic reaction and causes corrosion problems.
7. The effect of Li
Lithium plays the role of increasing aluminum alloy’s hardness, so Al-Li alloy is widely used in the aviation field. However, Li appeared along the grain boundaries, causing a rapid increase in the corrosion rate and local spread of corrosion.
Types of aluminum alloy corrosion
Uniform corrosion
Uniform corrosion is a common corrosion type that occurs when the pH is too high or too low. All alloy surface areas are eroded at the same rate. The aluminum oxide film can not protect the metal, and it will be eroded gradually.
Uniform corrosion can be easily identified and handled using paint or coatings at an allowable corrosion level.
With aluminum alloys, inhibitors like chromic acid or cathodic protection can be used.
Galvanic corrosion

Galvanic corrosion occurs when an aluminum alloy is connected to a conductive material, reacting more strongly in a conductive environment. At the contact point between the remaining aluminum and metal will form a corrosion attack. For example, in metal welds, corrosion will form concentrated on the less noble metal side.
Galvanic corrosion also occurs with heterogeneous aluminum alloys containing intermetallic compounds. For example, in aluminum alloys containing copper, corrosion is significantly increased if immersed in water or harsh environments.
In the case of aluminum and stainless steel contact with each other in a dry environment, the corrosion level will only increase slightly. But in a humid environment, it will increase markedly.
To prevent galvanic corrosion from occurring, it is necessary to separate the two metals from each other by inserting an insulating material such as neoprene rubber in the two metals’ contact position or by redesigning, so that the two metals do not touch each other.
Crevice Corrosion

Crevice corrosion originates from gaps or joints and then spreads to areas on the surface in a humid environment.
A typical example is the location where the bolt and the metal to which it is bolted form rust, in the presence of moisture or water entering the gap.
Pitting corrosion

Pitting corrosion is a form of local corrosion that occurs on a metal surface when it is soaked in a moisture environment.
Pitting corrosion usually occurs when the alloy surface is covered with a thin oxide film, which forms during metal manufacture or reaction with the environment.
Regarding aluminum alloy, the aluminum oxide film is formed very quickly, and the bonding creates a barrier between metal surfaces. However, this still does not prevent contact between moisture and holes on the metal surface.
When surface voids appear due to a local cell’s impact, these holes, if unable to mechanically repair themselves, will be filled with corrosive products that look like nodules.
Intergranular Corrosion
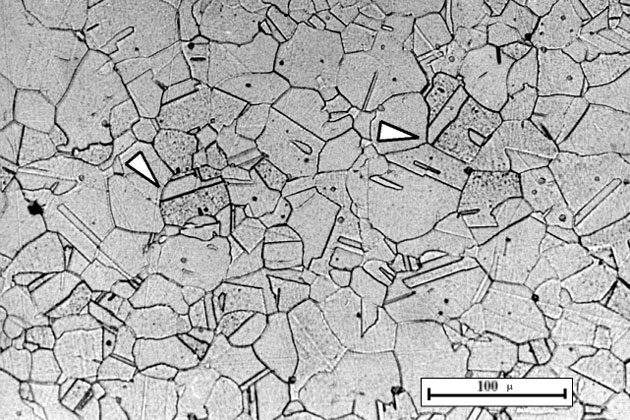
Intergranular corrosion is a local corrosion attack along the grain boundary or adjacent to the grain boundary of the metal, creating a corrosion path.
The grain boundary is a concentration of foreign particles, the deviation here causes it to be highly active than the inner area, so the corrosion rate here is faster.
The corrosion level can vary depending on its microstructure, which in turn depends on the heat treatment. Heat treatment produces particle precipitates and can make the grain boundary more active and rapidly destroy the material.
Exfoliation Corrosion
Exfoliation Corrosion is corrosion that appears along grain boundaries that run parallel to the metal surface. Compared to the base metal, the corrosive product is bigger in weight, which forces the metal to separate layers, causing the metal to swell.
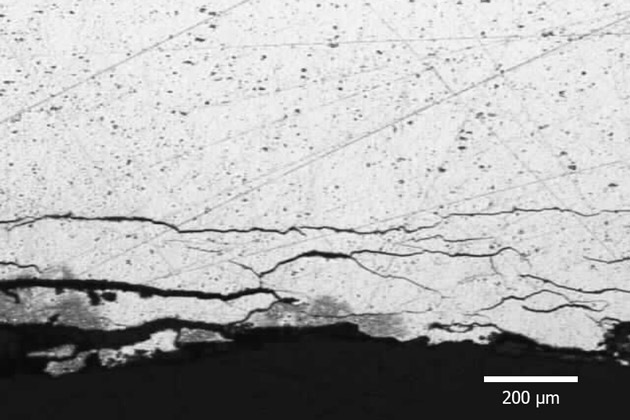
Exfoliation Corrosion usually happens in Al-Mg-Cu and Al-Zn-Cu alloys.
The extent of corrosion depends mainly on the composition and distribution of the precipitate at the grain boundary.
Stress Corrosion Cracking
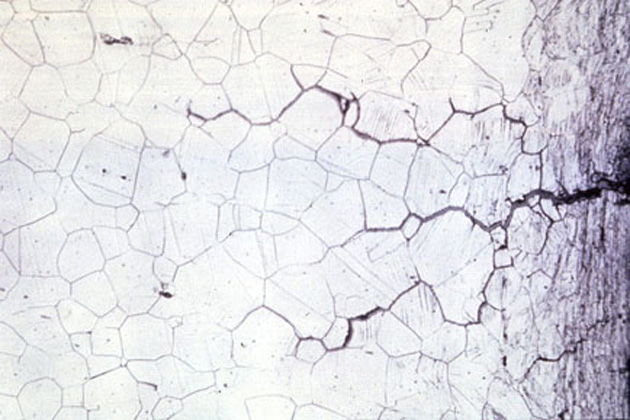
Stress Corrosion Cracking (SCC) is the deterioration of an alloy’s mechanical properties under the influence of stress and a corrosive environment. Initially, due to the mechanical stress, small cracks appear, then in the corrosive environment, the cracks develop very quickly, creating rapid destruction of the material.
Out of 8 aluminum alloys, 2xx.x, 5xx.x and 7xx.x series alloys are most susceptible to SCC.
The impetus of two agents: static tensile stress and the specific environment induces intergranular or transgranular cracking of the metal. SCC can happen unexpectedly and progress rapidly.
The specific environment is an essential factor in causing SCC. Only a very small concentration of some highly active chemicals can create a crack and gradually lead to catastrophic destruction on the alloy.
Corrosion Fatigue
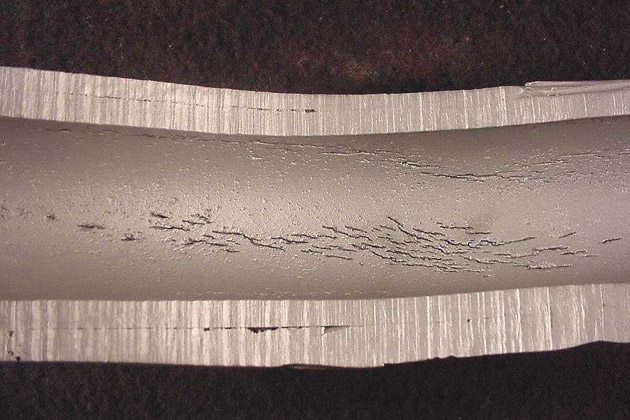
Corrosion Fatigue is the mechanical degradation of the material under the effects of stress and cyclic corrosion.
Although the aluminum surface has a naturally protected aluminum oxide film, this can be broken down when subjected to cyclic corrosive effects.
The fatigue strength of the material decreases through each cycle and does not depend on its metallurgical conditions.
Experimenting the corrosion resistance of aluminum alloy in NaCl, the fatigue strength is 108 cycles and its corrosion resistance is in the range of 25% to 35%, compared with that in the air.
Filiform Corrosion

Filiform corrosion is a special case of Crevice Corrosion, in which fine fibers appear like fine tunnels in random directions and without branching; these fine fibers contain corrosive products.
Filiform corrosion can occur on an unprotected metal surface or below the thin metal protective film, approximately 0.1mm thick. The film can be either a coating or protection against corrosion.
When the material comes into contact with water and oxygen, it causes corrosive products to penetrate into the space between the coating and the metal surface, especially through scratches, thereby gradually expanding into corrosive clusters.
Aluminum corrosion resistance
To effectively resist corrosion of aluminum alloys, it is necessary to isolate the metal surface from the environment completely. To achieve this, it is needed to use an organic coating such as paint.
However, painting on aluminum surface is not an easy process because aluminum’s surface does not have porosity. Therefore, it is needed to promote an oxide film on the surface through anodizing or conversion coating to improve the adhesion of the paint.
1. Create a coating on the aluminum surface
1.1. Anodising technique
The most common method to resist corrosion of aluminum and its alloys is anodizing. This is a method of creating a relatively thick oxide film outside the aluminum surface to help resist corrosion.
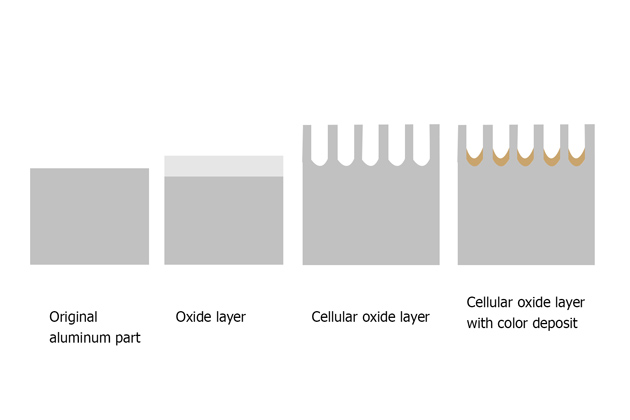
Inhibitors can be added to the outer layer of the anodized layer while this layer is forming, or it can also be added after formation to increase the level of metal protection.
There are different types of anodising:
- This is a popular and long-standing process to develop electrochemistry for the protective oxide film on the surface. An oxide film is formed by rapidly controlling the surface oxidation of aluminum. The film is relatively thin, from 0.5μm to 18μm, and does not conduct electricity.
- This method is more environmentally friendly than Chromic Anodising. It uses direct current and electrolyte solution, which is sulfuric acid, to oxidize the aluminum surface. The electric current passed through the aluminum surface’s oxygen release, forming a 1.8μm to 25 μm of the oxide layer. This process creates a tight layer of foam on top and needs sealing to close the pores.
- This method uses an aluminum alloy piece as an anode for electrolysis in a water environment containing sulfuric acid, and at least one compound is selected from the group of Molybdat, Wolfram, Vanadate, and Manganite. The maximum thickness of the oxide layer is 50μm.
- This method is quite similar to Sulfuric Acid anodizing, but it produces a thicker oxide layer that increases corrosion resistance. The oxide layer thickness is about 20 µm to 100 µm and is strictly controlled to avoid thermal deformation.
1.2. Chemical conversion coating

Chemical conversion coating is also known as a chemical film or chromate coating. This is the process of applying chromate to the metal substrate to create a corrosion-resistant, durable surface and has stable electrical conductivity.
This conversion coating is both a corrosion inhibitor and a primer for better adhesion to the topcoat.
To perform this procedure, it is necessary to immerse the metal part in the chemical containing the chrome compound for several minutes to form a film of the appropriate thickness. The chemical conversion coating is the film that is dry and hardens.
This process is described as follows:
Redox reaction between chrome and aluminum:
Cr6++ Al0 → Cr3+ + Al3+
Then react with hydroxide in water to create an alkaline solution:
Cr3+ + 3HO–→ Cr(OH)3
Al3++ 3HO–→ Al(OH)3
The alkaline solution dries and hardens, forming a dry coating, mainly Cr2O3, which is about 0.2-0.3μm thick.
However, this Chrome coating is quite toxic, so nowadays, people use alternative processing methods like self-assembled monolayers, sol-gel chemistries, rare earth, cobalt, etc. This is intended to protect against metal corrosion of surfaces that have been pre-eliminated IM particles.
2. Organic coatings

After the aluminum part is covered with the anode or chemically converted, the surface is ready to be coated with the organic coating. The organic coating system consists of a primer and a topcoat.
Primer is the main protective layer that will inhibit corrosion when it comes to water or metal contact. The topcoat will increase the level of protection and also be used for aesthetic purposes.
How to paint aluminum alloys: https://vietnamcastiron.com/painting-cast-aluminum-process/
Conclusion
From the studies of aluminum alloys based on the chemical basis, microstructure and environment, we can apprehend the crucial factors in aluminum alloy selection and development.
The microstructure determines the mechanical strength and the corrosion performance of the alloy. Gathering information on corrosion types as well as analyzing the corrosion resistance of aluminum alloys will enhance the study of surface treatment.
Increasing the corrosion resistance while ensuring a higher alloy strength requires more intensive research and testing. The current method of hardness enhancement, which simply precipitating the crystalline matrix, is no longer possible.
Read more about the most popular aluminum casting defect: https://vietnamcastiron.com/aluminum-casting-shrinkage/
This document was compiled by Mr.Dinh Tien Vu from VIC, based on following sources:
UK Aluminum industry Fact Sheet 2: Aluminum and Corrosion from Alfed.
Review Corrosion of Cast Aluminum Alloys from MDPI.
Durability and Corrosion of Aluminium and Its Alloys: Overview, Property Space, Techniques and Developments By N. L. Sukiman, X. Zhou, N. Birbilis, A.E. Hughes, J. M. C. Mol, S. J. Garcia, X.
Please cite the sources if you would like to use the information in this document.


